Co-Author: Josh Zetlin
This week the U.S. Food and Drug Administration (FDA) approved Epidiolex as a treatment for seizures associated with two rare and severe forms of epilepsy: Lennox-Gastaut syndrome and Dravet syndrome. Epidiolex, developed by GW Pharmaceuticals plc (Nasdaq: GWPH), a UK-based pharmaceutical company with U.S. operations, is the first FDA-approved drug made from a purified substance derived from the cannabis plant. Epidiolex is an oral solution of pure plant-derived cannabidiol or CBD. It is also the first drug approved in the United States for the treatment of patients with Dravet syndrome.
Today FDA approved the first drug with a compound derived from marijuana for treatment of seizures associated with certain rare, severe forms of epilepsy https://t.co/jXpMijNQKu pic.twitter.com/51i97XTm7l
— U.S. FDA (@US_FDA) June 25, 2018
Dravet syndrome, a rare genetic condition that appears during the first year of a child’s life, causes frequent fever-related seizures (febrile seizures). As a child with Dravet syndrome ages, other more-serious types of seizures typically occur. Some patients experience status epilepticus, a potentially life-threatening state of continuous seizure activity requiring emergency medical care. Children with Dravet syndrome usually experience poor development of language and motor skills, hyperactivity and difficulty relating to others. Anecdotally, many people found medicinal cannabis to be the most effective treatment for this debilitating condition.
As a pro bono matter, our firm currently represents the family of a child with Dravet syndrome before a local school district, advocating for the child’s right to use medicinal cannabis to control her seizures. We hope that Epidiolex may also provide relief for her and other children with this serious condition.
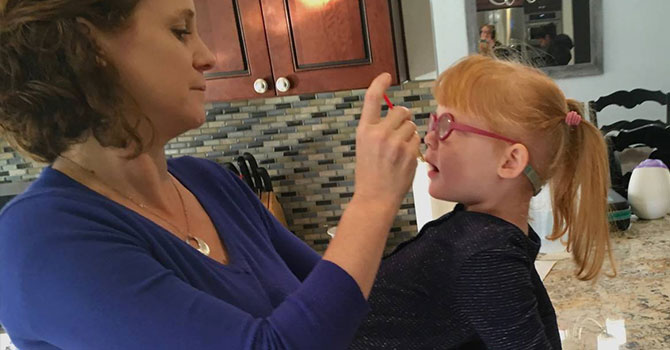
Jana Adams administers a tincture high in CBD to her daughter, Brooke, who has Dravet Syndrome. Source:(Lee Romney/KQED)
Under the U.S. Controlled Substances Act, CBD remains a Schedule I substance because it is a chemical component of the cannabis plant. In support of GW Pharmaceutical’s application for marketing approval with the FDA, the company conducted nonclinical and clinical studies to assess the abuse potential of CBD. While the FDA’s action does not change the legality of cannabis or CBD at the federal level (the FDA’s approval legalizes the prescription sale of just this drug, not CBD generally), the move is nevertheless encouraging.
Earlier FDA Approvals
Prior to this weeks’ approval of Epidiolex, the FDA approved several cannabinoid-based medicines derived from isolated synthetics, specifically the drugs Marinol, Syndros and Cesamet.
Both Marinol and Syndros include the active ingredient dronabinol, a synthetic delta-9- tetrahydrocannabinol (THC), and provide treatment for anorexia associated with weight loss in patients with AIDS and nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments. It has been suggested that Syndros could generate peak annual sales of up to $400 million. Cesamet includes the active ingredient nabilone, which is synthetically derived and has a chemical structure similar to THC. According to its developer, Meda Pharmaceuticals, Inc., Cesamet is a purely man-made synthetic drug that activates the cannabinoid receptor CB1, which reduces proemetic signaling in the vomit center and thus inhibits nausea.
Other Cannabis-Derived Drugs
GW Pharmaceuticals, the developer of Epidiolex, previously developed the world’s first prescription medicine derived from the cannabis plant, a drug named Sativex. While Sativex lacks FDA approval, it is approved throughout Europe and in many other key markets such as Canada, Australia and Brazil as a treatment of spasticity caused by Multiple Sclerosis. Sativex also received approval in Israel for both MS spasticity and pain as well as for chronic cancer pain. According to GW Pharmaceutical’s website, Satlivex is an oromucosal spray of a formulated extract of the cannabis sativa plant that contains the principal cannabinoids delta-9-tetrahydrocannabinol (THC) and CBD in a 1:1 ratio, as well as specific minor cannabinoids and other non-cannabinoid components. GW Pharmaceuticals also has a pipeline of other clinical stage cannabinoid product candidates for neurological conditions, such as autism spectrum disorders and schizophrenia.
Worldwide there are several other drug developers working on potential therapeutics based upon cannabinoids. For example, Corbus Pharmaceuticals, a drug developer located in Massachusetts, is working on a new drug called Anabasum. This synthetic oral endocannabinoid-mimetic drug binds to the CB2 receptor expressed on activated immune cells and fibroblasts. Corbus has reported positive mid-stage results of the drug in patients with systemic sclerosis and cystic fibrosis.
Cara Therapeutics, located in Stamford, Connecticut, is a traditional drug developer trying to expand its products to include cannabinoid-based drugs. The most developed product in Cara’s pipeline is CR845, a kappa opioid receptor agonist (KORA). This experimental drug is designed to reduce the central nervous system side effects, like pain and itching, often observed with traditional opioids, such as morphine.
Zynerba Pharmaceuticals is a company wholly dependent on cannabinoid therapies. Zynerba has two drugs currently in development. The first, called ZYN001 for testing purposes, is getting ready for mid-stage trial initiation in the second half of this year as a treatment for fibromyalgia and peripheral neuropathic pain. ZYN001 is a THC pro-drug patch that the company believes could have future uses for treating chronic pain and gastrointestinal disorders. The second, called ZYN002, is a CBD-based gel that is absorbed through the skin, which the company is testing as a potential treatment for adult epilepsy and osteoarthritis.
In addition to pharmaceutical companies, academic institutions are beginning to focus on the therapeutic potential of cannabis. For example, UCLA recently formed the Cannabis Research Initiative, an academic program dedicated to studying health benefits and risks of cannabis on the body, brain and mind, as well as the wide-ranging health, legal, economic, and social impacts of cannabis. These developments all reflect growing recognition of the vast pharmaceutical promise of the cannabis plant.



